High-Performance Liquid Chromatography (HPLC) is a cornerstone analytical technique in many scientific disciplines, including pharmaceuticals, environmental testing, and food safety. A crucial aspect of HPLC is the retention time of analytes, which refers to the time it takes for a particular compound to travel through the column and be detected. Retention time variability can lead to inaccurate results, making it essential for HPLC professionals to understand its causes and how to diagnose and rectify them. This article will explore the factors contributing to retention time variability, the implications of these fluctuations, and effective troubleshooting strategies.
Understanding Retention Time
Retention time (RT) is influenced by various factors, including the nature of the analyte, the mobile phase composition, the column characteristics, and the operational parameters of the HPLC system. Ideally, RT should remain consistent across repeated analyses, as this consistency allows for accurate quantification and identification of compounds. Variability in RT can complicate method validation and impact the reproducibility of results.
Common Causes of Retention Time Variability
- Column Characteristics
- Column Age and Condition: Over time, columns can become less efficient due to stationary phase degradation or particulate buildup. An aged or damaged column can lead to inconsistent retention times.
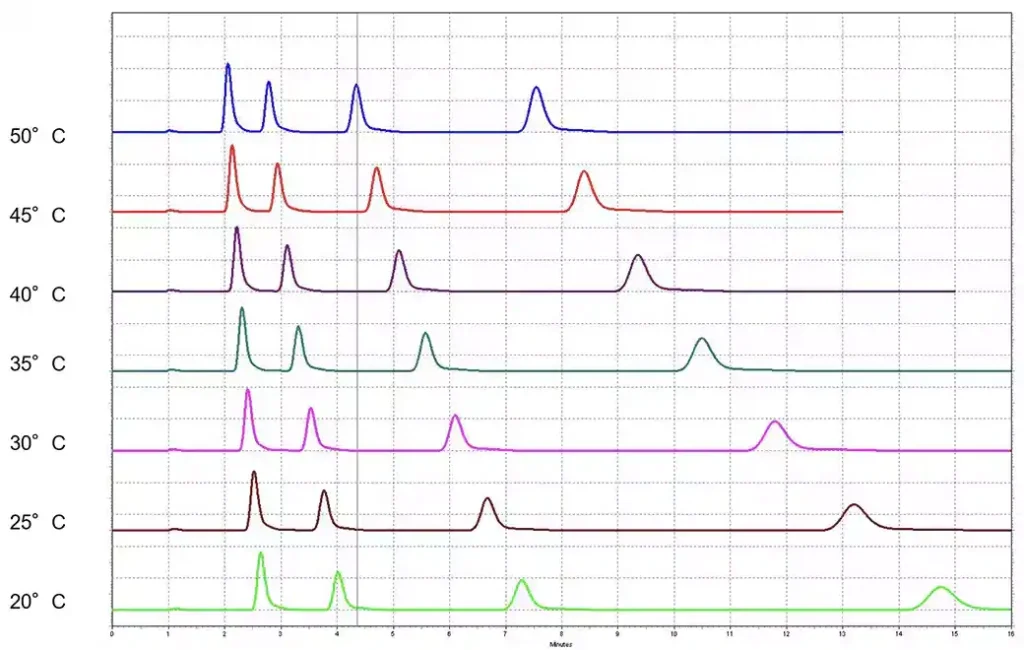
- Column Temperature: Variations in column temperature can significantly affect retention times. Even small temperature fluctuations can alter the viscosity of the mobile phase and the interaction between the analyte and stationary phase.
- Mobile Phase Composition
- Solvent Quality: Impurities in solvents can introduce variability. Ensure solvents are high purity and free from contaminants.
- pH Changes: The pH of the mobile phase can affect the ionization state of analytes, altering their retention behavior. Variations in pH can lead to significant changes in RT.
- Buffer Concentration: Inconsistent buffer concentrations can lead to variability in retention times, especially for ionic compounds.
- Injection Technique
- Sample Volume and Composition: Variability in sample volume or changes in the matrix can influence retention times. A consistent and appropriate sample injection volume is crucial for reproducibility.
- Injection Port Contamination: Residues left in the injection port from previous runs can affect subsequent injections, leading to variability in retention times.
- Pump Performance
- Flow Rate Fluctuations: Inconsistent flow rates can cause variations in retention times. It’s essential to regularly check and calibrate the pump.
- Air Bubbles: The presence of air bubbles in the mobile phase can lead to fluctuations in flow rates, affecting retention times.
- Detector Response
- Detector Calibration: If the detector is not calibrated correctly, it can lead to erroneous retention time readings. Regular calibration is essential for accurate results.
- Sensitivity Variations: Changes in detector sensitivity over time can affect the apparent retention times of analytes.
Diagnosing Retention Time Variability
When facing retention time variability, a systematic approach to diagnosis can help identify the underlying issues. Here are steps to effectively diagnose and resolve these problems:
- Review Historical Data
- Start by reviewing historical retention time data for the analytes of interest. Identify any patterns or trends in variability. Look for changes in retention times over time or under specific conditions.
- Evaluate Column Conditions
- Inspect the HPLC column for signs of damage or degradation. A significant change in retention time might indicate that the column needs replacement. Perform a column performance test, such as checking theoretical plates and symmetry.
- Monitor Temperature
- Ensure that the column temperature is stable and set according to method requirements. Utilize a column oven if necessary to maintain consistent temperatures during analysis.
- Check Mobile Phase Composition
- Verify the quality and composition of the mobile phase. Use fresh solvents, and ensure that they are free from contaminants. If buffers are used, confirm their pH and concentration.
- Assess Injection Techniques
- Ensure that the sample injection technique is consistent. Use an autosampler if possible, and monitor for carryover or contamination in the injection port. Run blanks between samples to check for carryover.
- Evaluate Pump Performance
- Check the pump for any signs of malfunction, such as irregular flow rates or pressure fluctuations. Regular maintenance and calibration of the pump are essential to avoid flow inconsistencies.
- Inspect Detector Calibration
- Regularly calibrate the detector to ensure accurate readings. Use standard solutions to confirm the detector’s performance and consistency.
- Run System Suitability Tests
- Conduct system suitability tests before routine analyses. This includes checking retention times, peak resolution, and other performance parameters to ensure the system is functioning optimally.
- Implement Quality Control Measures
- Establish quality control (QC) measures, such as running control samples or standards periodically. This practice will help identify when retention times deviate from expected values.
- Document and Analyze Changes
- Maintain a log of all changes made to the HPLC system, including maintenance, solvent changes, and method modifications. Analyzing this information can help identify trends associated with retention time variability.
Implications of Retention Time Variability
Retention time variability can lead to several significant issues, including:
- Inaccurate Quantification: Fluctuations in retention time can affect the accuracy of quantifying analytes, leading to potential errors in concentration calculations.
- Failed Method Validation: For regulated industries, consistency in retention time is critical for method validation. Variability can lead to non-compliance with regulatory standards.
- Increased Analysis Time: If retention times are not consistent, it can lead to longer analysis times as methods may need to be repeated to achieve reliable results.
- Loss of Confidence in Results: Variability can undermine confidence in analytical results, impacting decision-making in research and quality control processes.
Diagnosing and resolving retention time variability in HPLC is crucial for maintaining the integrity of analytical results. By understanding the underlying causes and implementing systematic troubleshooting strategies, HPLC professionals can enhance the reliability and reproducibility of their analyses. Regular maintenance, careful monitoring of conditions, and thorough documentation are essential practices that contribute to effective HPLC operation.
In a field where precision is paramount, addressing retention time variability not only improves analytical performance but also upholds the credibility of the results generated. By taking proactive measures and cultivating a deep understanding of the HPLC system, professionals can ensure their analyses remain consistent, accurate, and compliant with industry standards.




