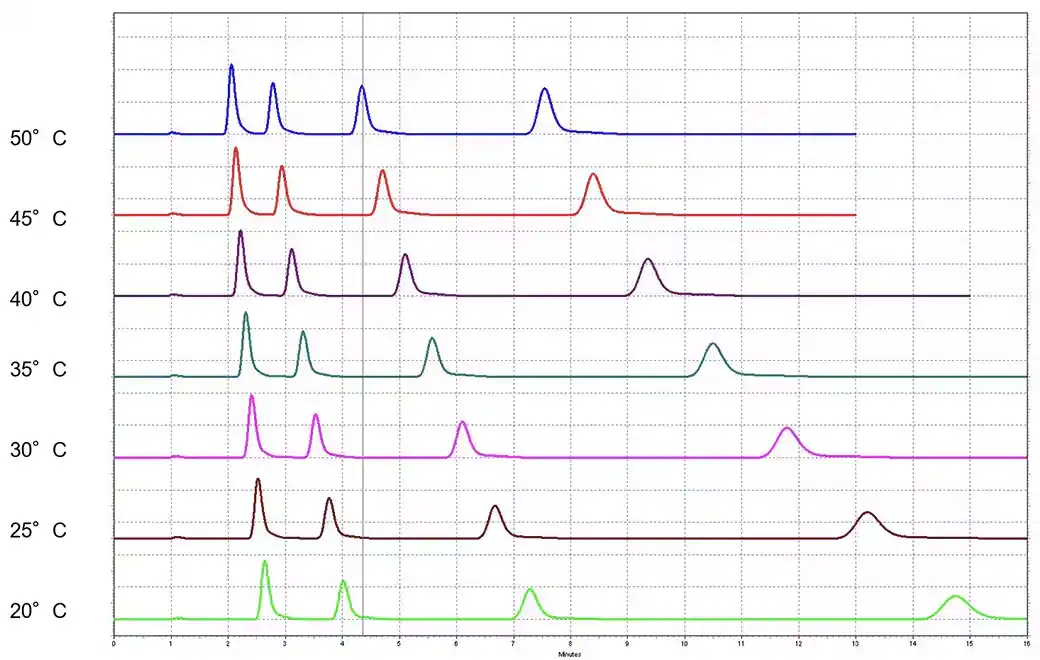
High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique widely used in laboratories for various applications, including pharmaceuticals, environmental monitoring, and food testing. One of the critical components of HPLC method validation is the System Suitability Test (SST), which ensures that the HPLC system is functioning properly and producing reliable results. This article will guide you through the process of performing a System Suitability Test in HPLC, covering its importance, parameters to assess, and step-by-step procedures.
What is a System Suitability Test?
A System Suitability Test is a series of tests performed on an HPLC system to verify that it is operating correctly and is suitable for the intended analysis. The SST evaluates the performance characteristics of the system before running samples to ensure consistent and reliable results. The parameters tested during SST are essential to establishing method validity and reproducibility.
Importance of System Suitability Testing
- Quality Assurance: SST acts as a quality control measure to confirm that the system is functioning within specified limits.
- Regulatory Compliance: Many regulatory bodies, including the FDA and ICH, require SST as part of method validation for pharmaceuticals.
- Consistency and Reproducibility: Regular SSTs help in ensuring consistent results across multiple analyses and over time.
Key Parameters for System Suitability Testing
Several key parameters should be assessed during a System Suitability Test. These typically include:
- Retention Time: Measures the time it takes for a compound to elute from the column. It should be consistent within specified limits.
- Theoretical Plates (N): A measure of column efficiency; higher values indicate better separation performance.
- Tailing Factor (Tf): Assesses peak shape. A value close to 1 indicates a symmetrical peak.
- Resolution (Rs): The ability to separate two peaks. A resolution of 1.5 or greater is often considered acceptable.
- Signal-to-Noise Ratio (S/N): Determines the sensitivity of the method. Higher ratios indicate better detection limits.
Step-by-Step Guide to Performing a System Suitability Test
Step 1: Prepare the HPLC System
Before conducting the SST, ensure that the HPLC system is properly set up and calibrated:
- Column Installation: Ensure the column is correctly installed and equilibrated with the mobile phase.
- Mobile Phase Preparation: Prepare the mobile phase according to the method specification, filtering it to remove any particulates.
- Temperature Settings: Set the column oven to the appropriate temperature for your method.

Step 2: Prepare the Standard Solution
To perform the SST, you will need a standard solution that contains the analytes of interest at known concentrations:
- Concentration Selection: Prepare a standard solution that reflects the expected concentration of your samples.
- Filtration: Filter the standard solution to eliminate any particulates that could interfere with the analysis.
- Stability Check: Ensure the standard solution is stable and freshly prepared to avoid degradation.
Step 3: Run the System Suitability Test
- Inject the Standard Solution:
- Start by injecting the standard solution into the HPLC system.
- Use a consistent injection volume as specified in your method (typically 10-20 µL).
- Record Chromatograms:
- Collect chromatographic data for the injected standard solution.
- Ensure the system has reached equilibrium before starting the injections.
Step 4: Evaluate the Results
Once you have the chromatograms, evaluate the key parameters:
- Retention Time:
- Check that the retention time of your analytes falls within acceptable limits as defined in your method.
- Theoretical Plates (N):
The number of theoretical plates (N) can be calculated using the formula:
N = (tR)2 / (tR – tM)2
where, tR is the retention time of the analyte and tM is the retention time of an unretained compound (void time). - Tailing Factor (Tf):
The tailing factor (Tf) can be calculated using the formula:
b = distance from the peak maximum to the back edge (tail) of the peak.
Tf = b / a
where:- a = distance from the peak maximum to the front edge of the peak.
- Resolution (Rs):
The resolution (Rs) between two peaks can be calculated using the formula:
tR1 = retention time of the first peak.
Rs = 2(tR2 – tR1) / (w1 + w2)
where:- tR2 = retention time of the second peak.
- w1 = width of the first peak at its baseline.
- w2 = width of the second peak at its baseline.
- Signal-to-Noise Ratio (S/N):
- Measure the height of the analyte peak and the baseline noise to calculate the S/N ratio.
Step 5: Compare Results Against Acceptance Criteria
Each of the parameters evaluated should be compared against the established acceptance criteria:
- Retention Time: Must be consistent with previously established values.
- Theoretical Plates (N): Should meet or exceed the minimum requirement set by the method (generally 2000< is acceptable).
- Tailing Factor (Tf): Should be close to 1 for symmetrical peaks (generally <2 is acceptable).
- Resolution (Rs): Must meet the method requirements (typically >1.5).
- Signal-to-Noise Ratio (S/N): Should be above the method’s threshold for quantitation.
Step 6: Document Findings
Documentation is crucial in any analytical method. Ensure to record:
- Date and Time of SST
- Preparation and Injection Details
- Chromatograms
- Results for Each Parameter
- Any Deviations and Corrective Actions Taken
This documentation is vital for regulatory compliance and future reference.
Step 7: Address Any Deviations
If any of the SST parameters do not meet the established criteria, investigate and address the issues:
- Identify the Source of the Problem: Review sample preparation, mobile phase, and column condition.
- Perform Maintenance: If necessary, clean or replace parts of the HPLC system.
- Retest: Once issues are resolved, repeat the SST to confirm system suitability.
Performing a System Suitability Test is a vital step in ensuring that your HPLC system is functioning optimally. By evaluating key performance parameters and adhering to established acceptance criteria, you can ensure the reliability of your analytical results. Regular SSTs not only enhance the quality of your analysis but also contribute to regulatory compliance and overall laboratory efficiency. Always document your findings and be prepared to troubleshoot any issues that arise to maintain the integrity of your HPLC analyses. Happy testing!